Publishing Automation for Medical Device Regulatory Submissions
Reduce Manual Effort, Rework, Errors, Cycle Time, and Cost
Regulatory document publishing is a critical process for medical device companies. While many organizations optimize it using best practices, outsourcing, or various tools, most steps still involve manual effort. This can lead to rework, extended cycle times, compliance risks, staff burnout, and increased costs.
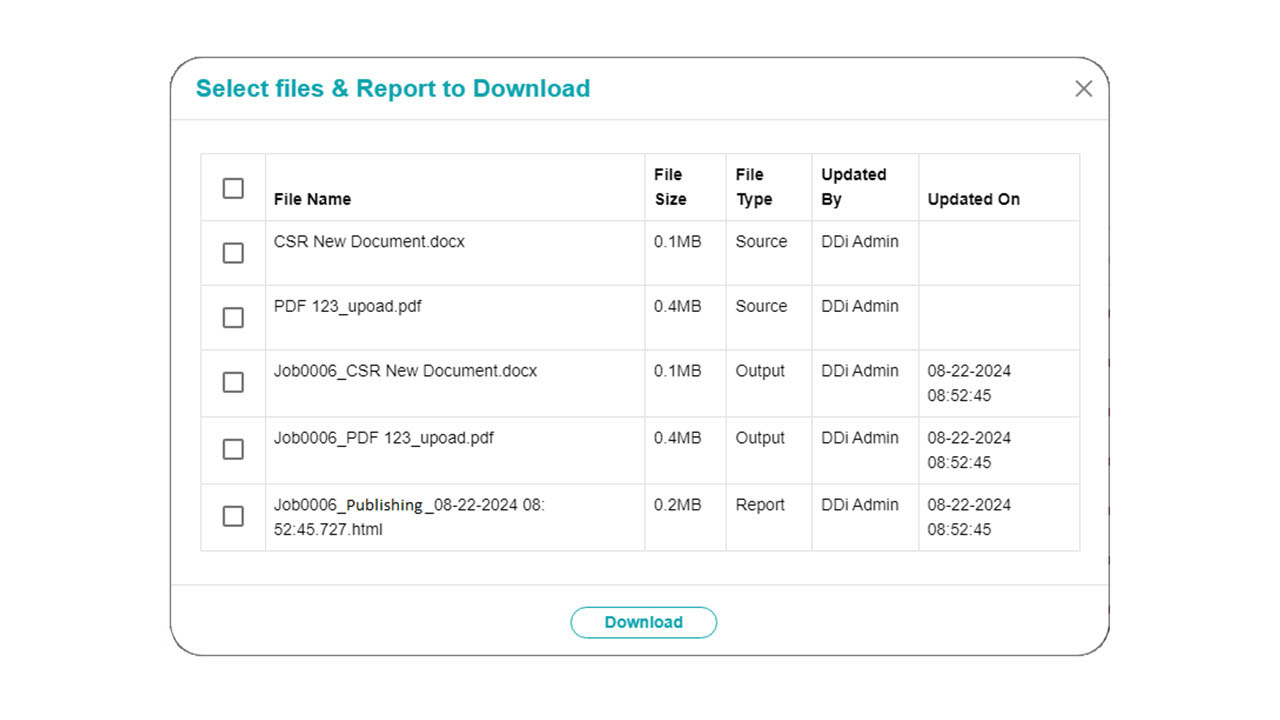
Our Publishing Automation Solution streamlines document publishing for regulatory submissions, ensuring compliance with global medical device regulations (FDA, EU MDR, etc.) without disrupting your existing submission infrastructure. Whether your source files are in MS Word or PDF, our solution delivers submission-ready files in minutes. With a library of 400+ publishing rules, you can create custom plans tailored to your documents, applying rules as needed. The solution seamlessly integrates into your existing systems, whether on-premise, cloud-based, or plugin-driven.
Time & Cost Savings: Achieve 70%+ reduction in document publishing costs. Guaranteed!
Pre-Publishing (Document Level)
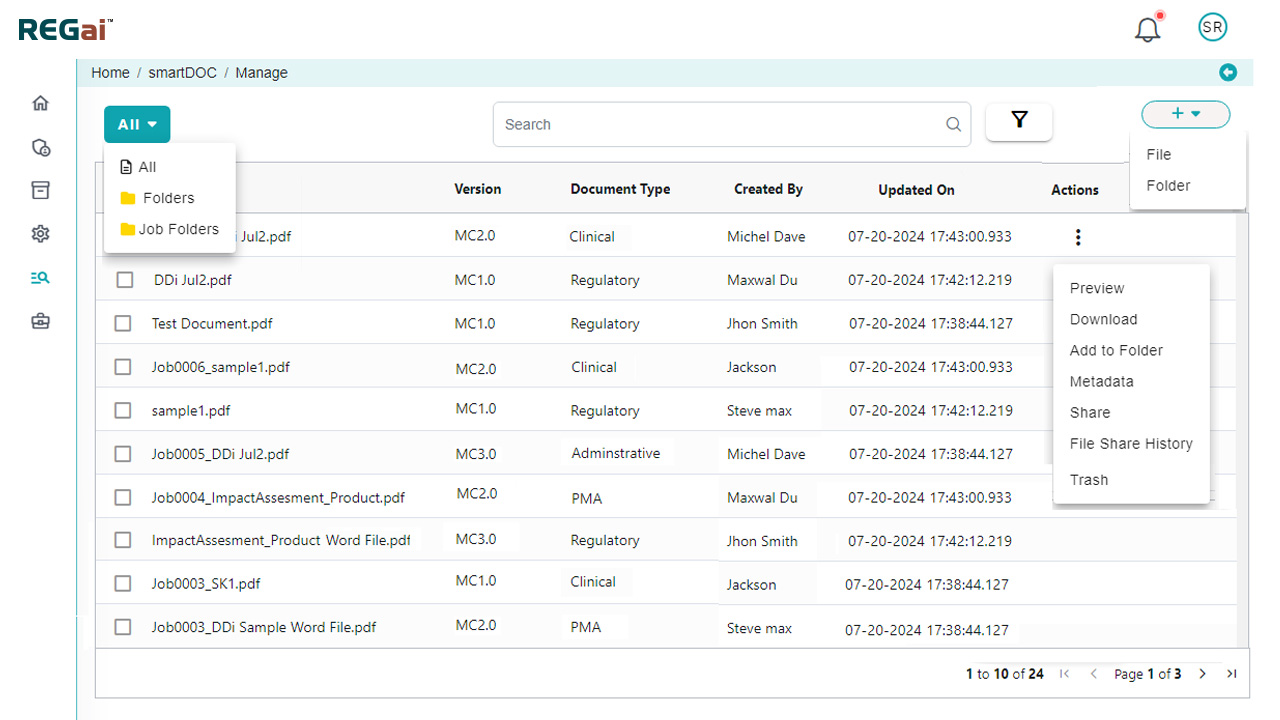
- Publishing Rules: Out-of-the-box compliance rules for FDA, EU MDR, IVDR, and other global medical device regulations. Leverage custom publishing rules from our extensive library for MS Word and PDF, covering:
- Tables, font management, TOC, bookmarks, headers/footers, hyperlinks
- File-level general options and more
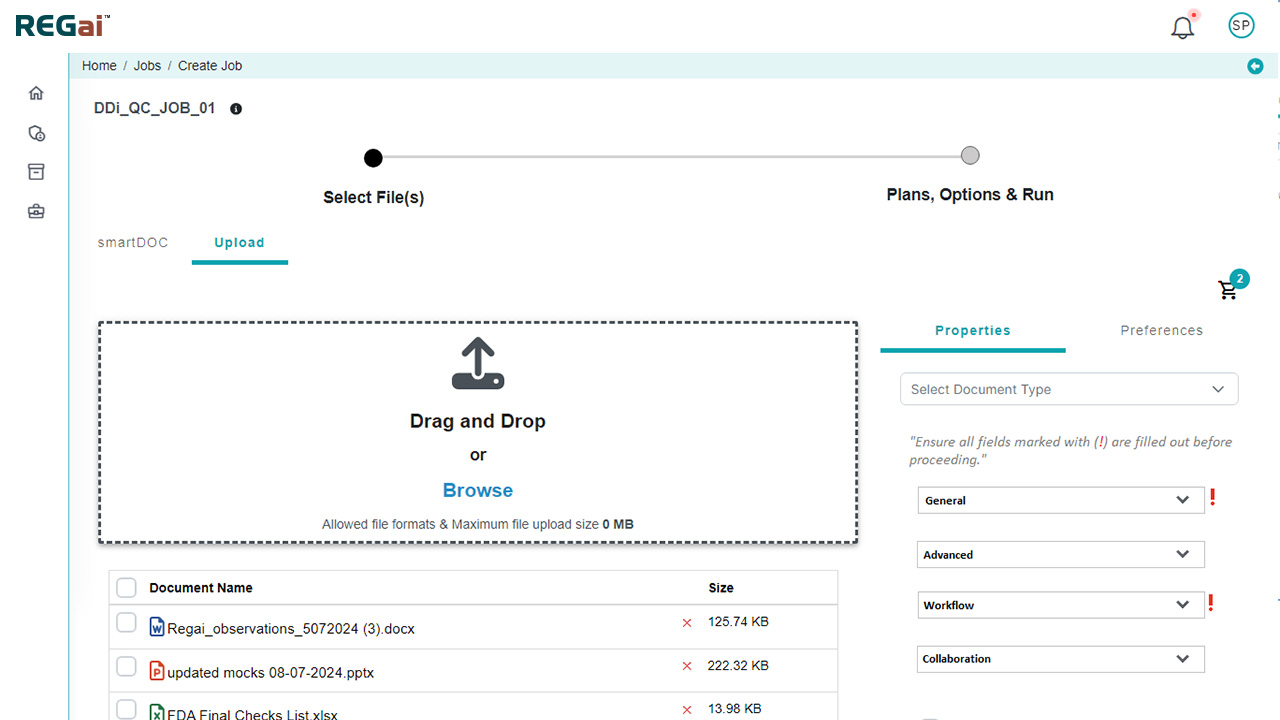
- How to Use: Three flexible options:
- Word Plugins – Apply automation directly within MS Word
- Standalone Tool – Upload files from your desktop/EDMS for processing
- Automated Publishing – Connect to your existing document repositories for scheduled batch publishing (hourly, daily, or custom schedules)
- Seamless Integration: Pre-built interfaces connect with your current eCTD systems, RIM, EDMS, ERP, SharePoint, Documentum, BOX, and other third-party platforms used in medical device regulatory workflows.
Post-Publishing Automation
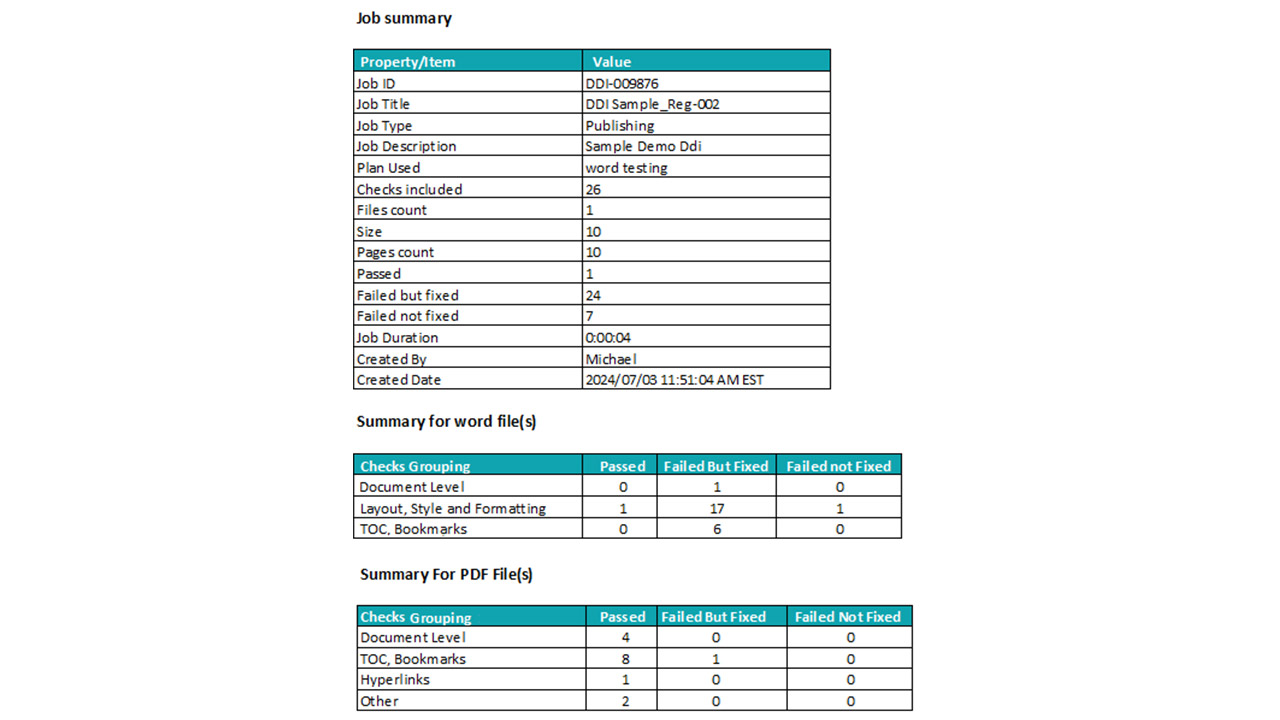
Enhance compliance by automating dossier validation and regulatory checks:
-
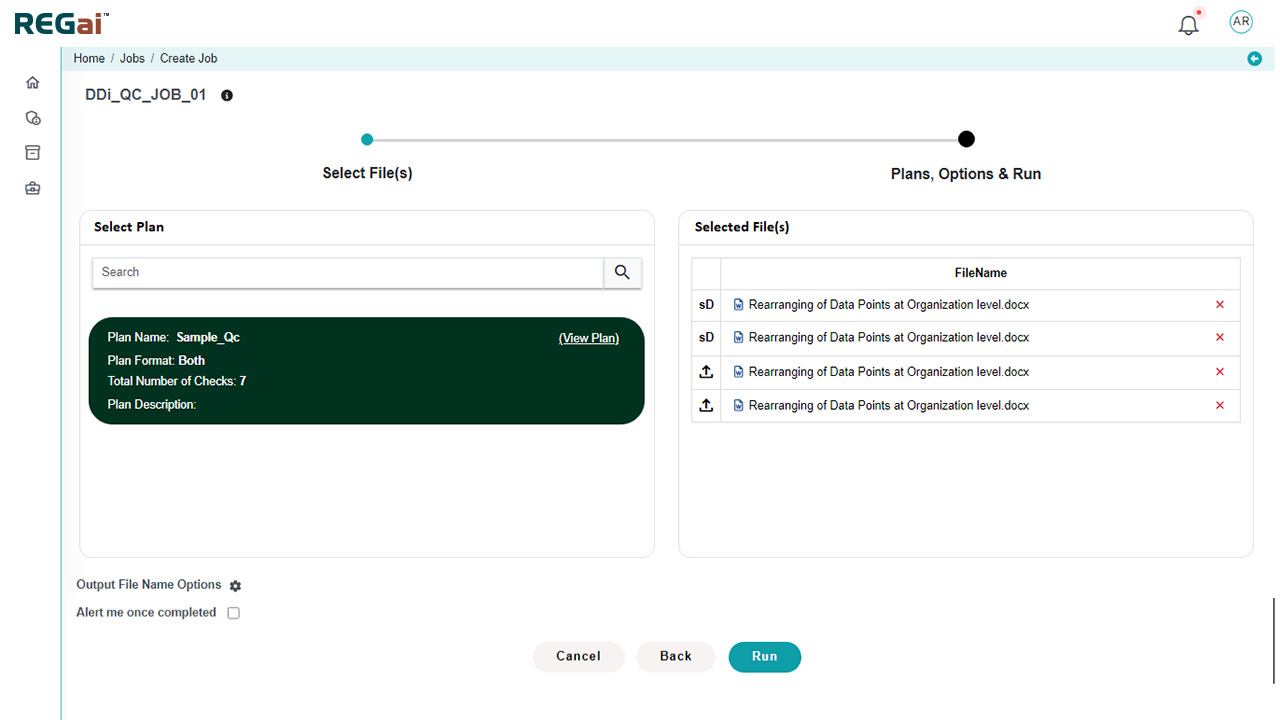
Automate validation based on pre-defined compliance checklists
-
Custom validation plans at the dossier level, with different checks for individual documents
-
Inter-document hyperlink validation and automated updates
-
Auto-update of file names, elements, and leaf titles to align with internal compliance standards
-
Selective validation path testing for defined regulatory checks
We’re Here To Help
Get in touch with us
Let's talk about how DDi can help you

