Regulatory Project Management & Tracking for Medical Devices
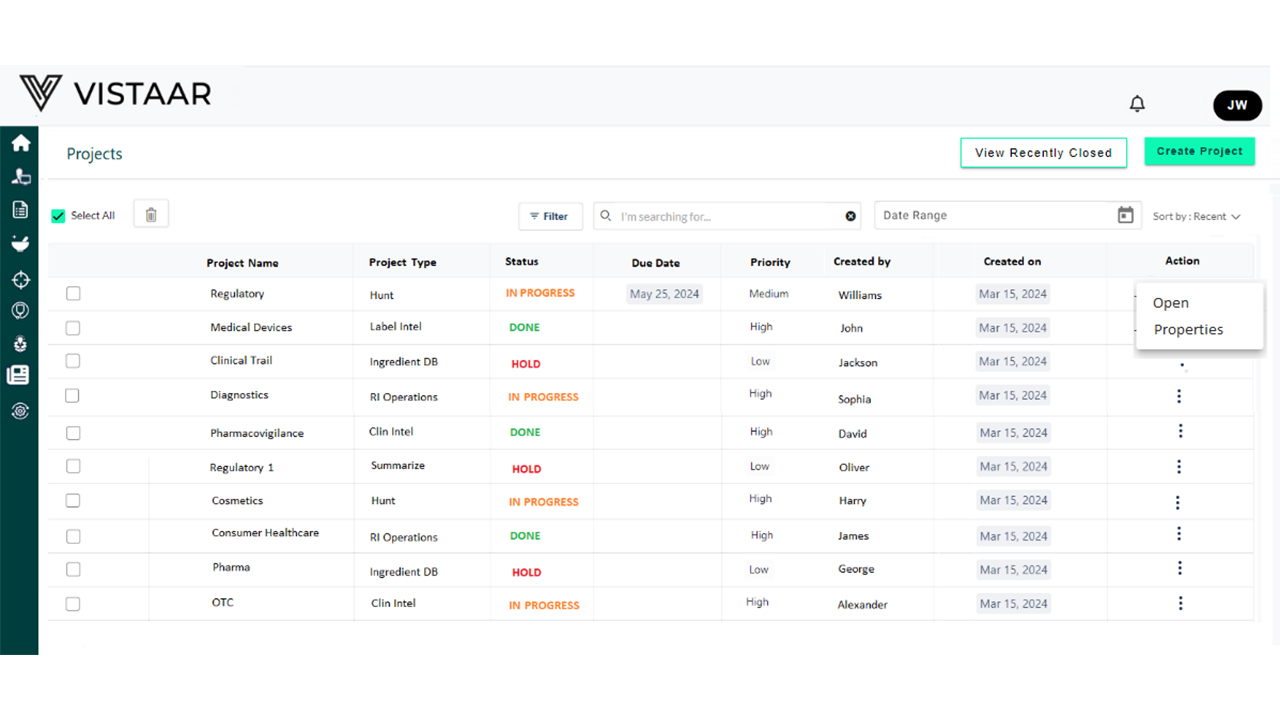
Regulatory project management in our solution supports the planning, organization, and management of medical device regulatory projects, ensuring efficient tracking to meet compliance deadlines and budget constraints. The goal is to streamline regulatory processes and maintain compliance with global medical device regulations. It facilitates coordination and communication among stakeholders (both internal and external, including those without system access), such as regulatory authorities, project teams, and partners, to ensure the successful completion of regulatory projects.
Our Regulatory Project Management solution for medical devices includes:
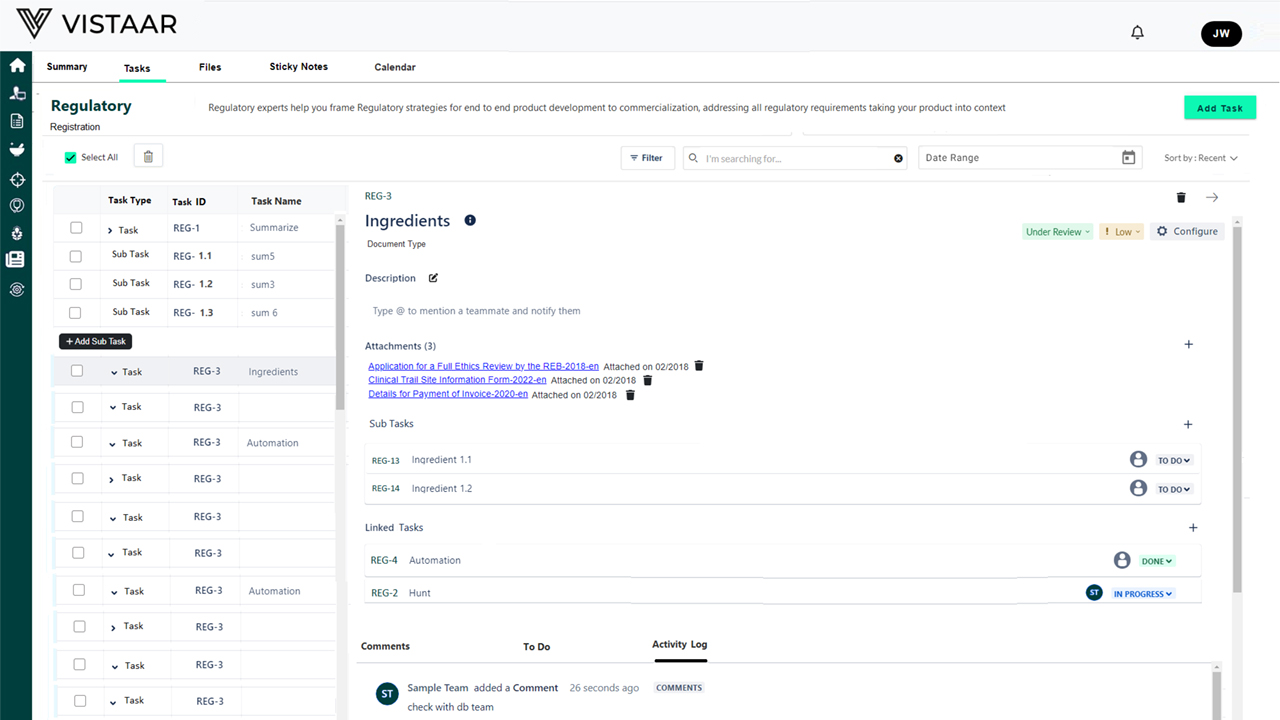
- Planning: Creating a structured project plan that defines scope, objectives, timelines, and budget to meet regulatory submission and compliance requirements.
- Risk assessment: Identifying potential regulatory risks that may impact project timelines and implementing risk management strategies to mitigate compliance challenges.
- Stakeholder Engagement: Engaging with key stakeholders, including regulatory bodies (FDA, EU MDR, etc.), internal teams, and third-party partners, to ensure alignment with compliance expectations. The built-in workflow management feature streamlines approvals and communications.
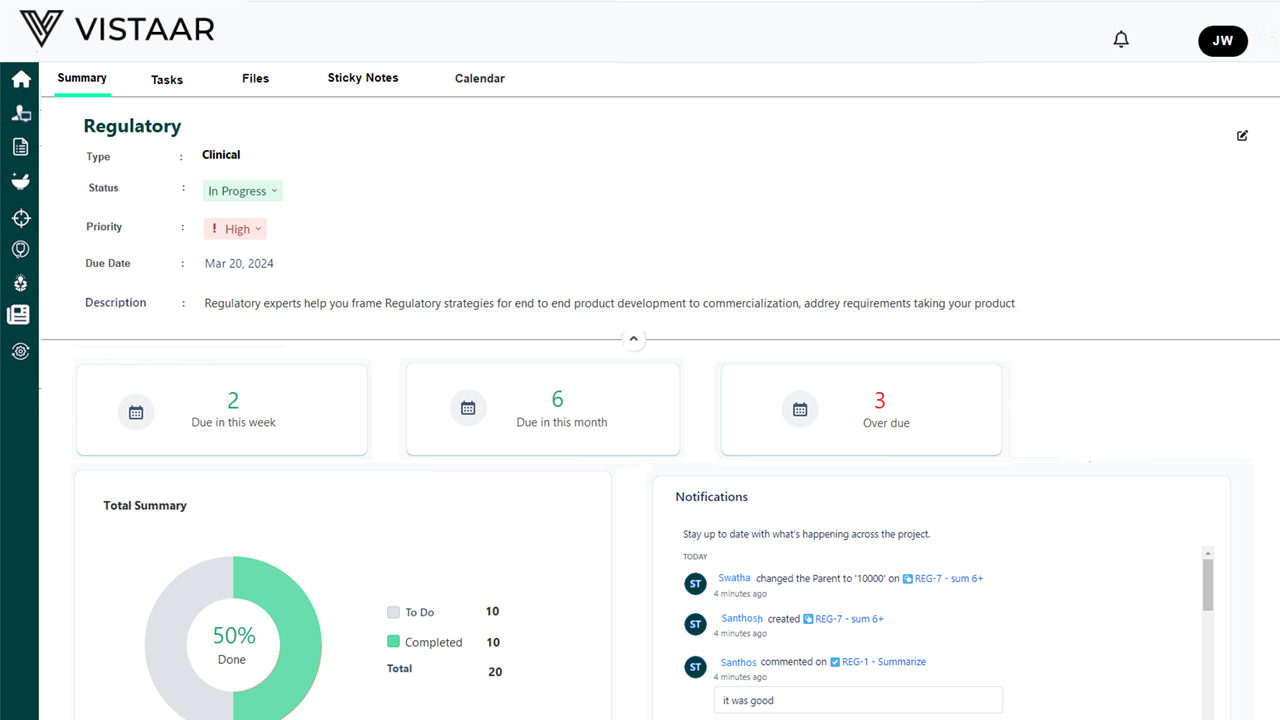
- Monitoring and Evaluation: Continuously tracking project progress against the plan, identifying deviations, and taking proactive corrective actions. Automated system alerts help maintain compliance readiness.
- Reporting: Generating and delivering regular progress reports to stakeholders, including regulatory agencies, internal teams, and external partners, ensuring transparency and regulatory alignment.
- Dashboards: Customized dashboards provide an intuitive interface for tracking project status, regulatory milestones, and submission timelines.
- Security: The system enforces role-based and group-based security controls, ensuring appropriate access levels within teams and organizations to safeguard regulatory data.
This solution simplifies the regulatory project lifecycle for medical device companies, ensuring compliance, efficiency, and effective tracking of submissions and approvals.
We’re Here To Help
Get in touch with us
Let's talk about how DDi can help you


