Regulatory Impact Assessment (RIA)
Requests and queries to Regulatory teams are increasing daily and most of those teams need a short/quick turn-around pressure on Regulatory function. Regulatory Impact Assessment (RIA) helps to have base decisions on regulatory facts plus evidences. It critically assesses the positive and negative effects of proposed (Guidance) and existing regulations and non-regulatory alternatives.
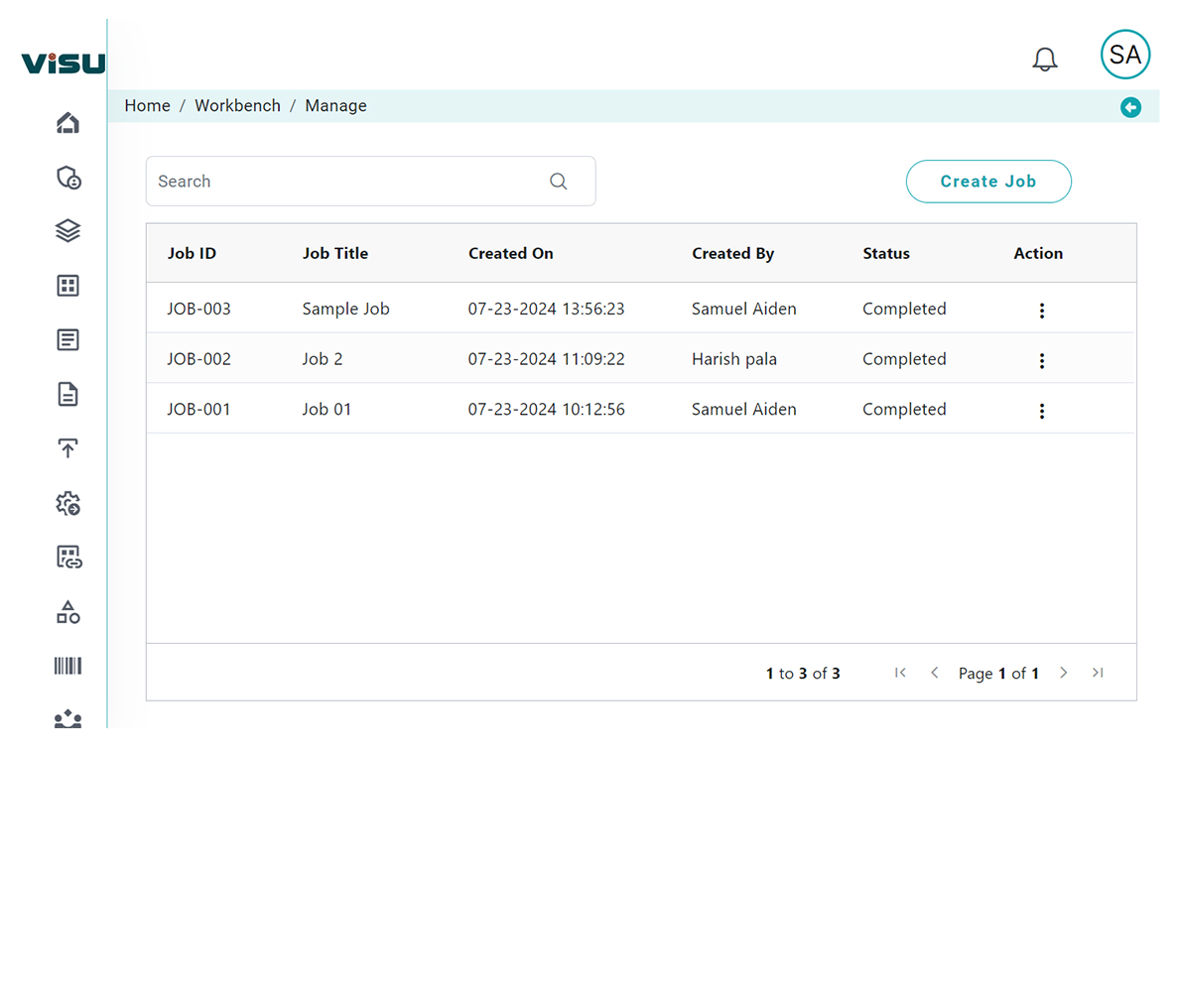
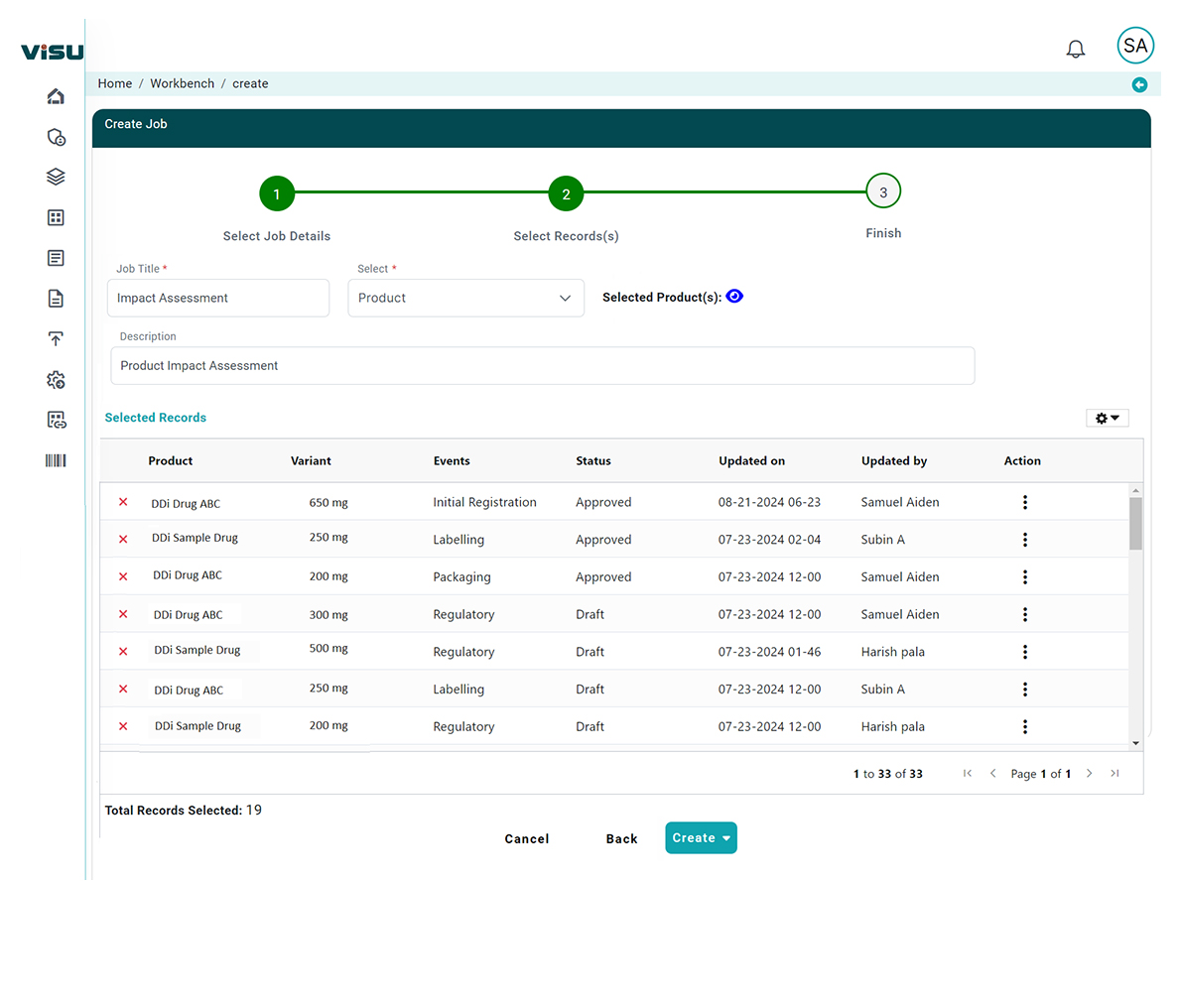
The purpose of RIA is to assess the impact and help drive a pathway. With our assessment module, Regulatory teams can cut down cycle (waiting) time and feel less burdened clearing their change management and new regulation impact assessment “to-do” lists.
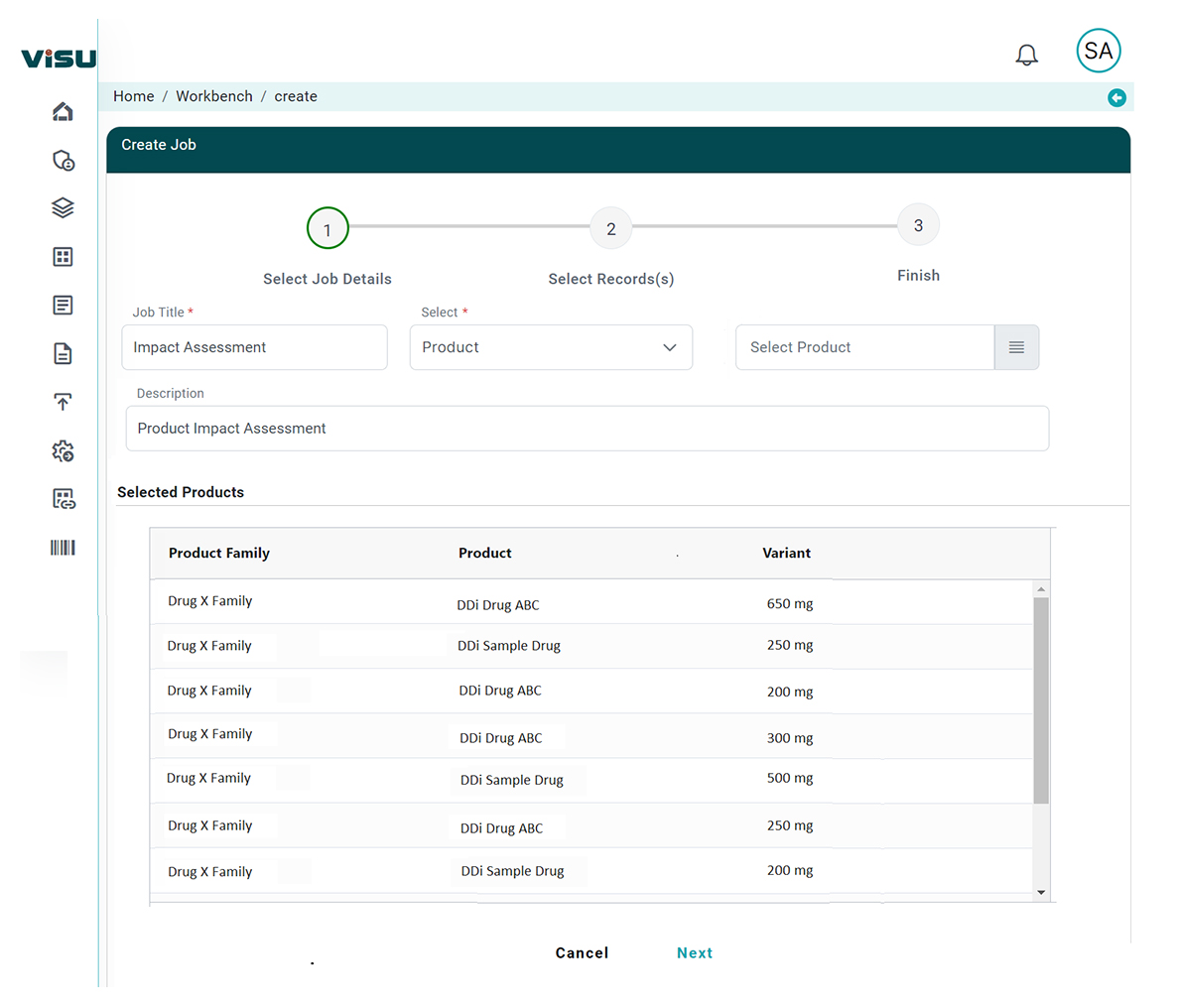
Our RIA typically involves functionality including:
- Identification: Identifying the key area the regulation is intended to address.
- Strategy development: Developing a range of options to address the change.
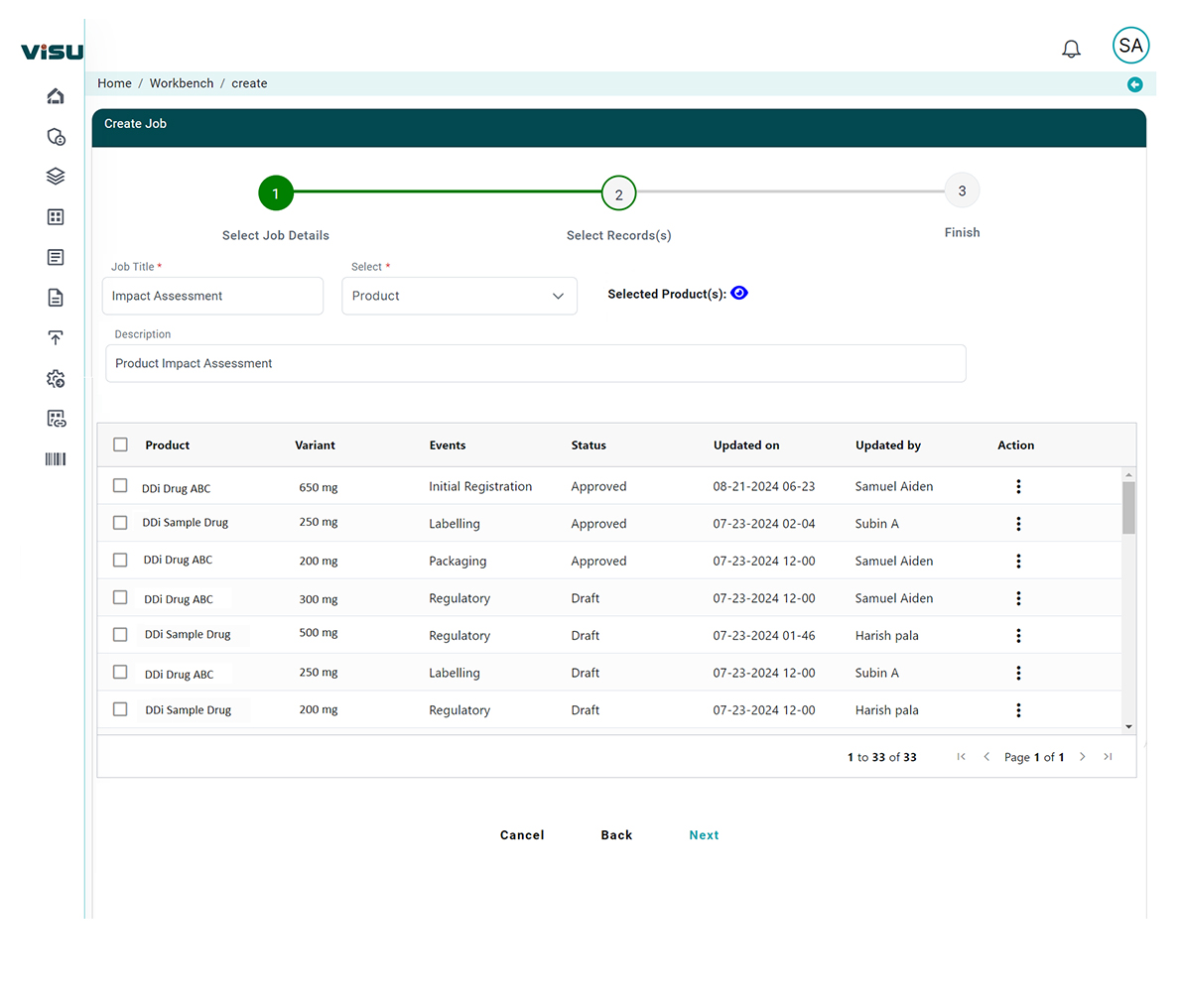
- Impact analysis: Assessing the potential impacts of each option, including the business and patient safety impacts.
- Consultation: Consulting with stakeholders and affected parties to gather feedback on the proposed options.
- Decision-making: Selecting the preferred option based on the analysis and consultation.
- Monitoring (Project Monitoring) and review: Monitoring the implementation of the regulation and reviewing its impact over time.
Our RIA solution is a valuable for Regulatory teams where it can help them identify the most effective and efficient options while minimizing risks.
We’re Here To Help
Get in touch with us
Let's talk about how DDi can help you