Submission & Publishing Management
Regulatory operations publishing involves the compilation, preparation, and submission of regulatory documents to health authorities or notified bodies to support the approval and maintenance of products. Our solution supports following regulatory operations publishing areas:
-
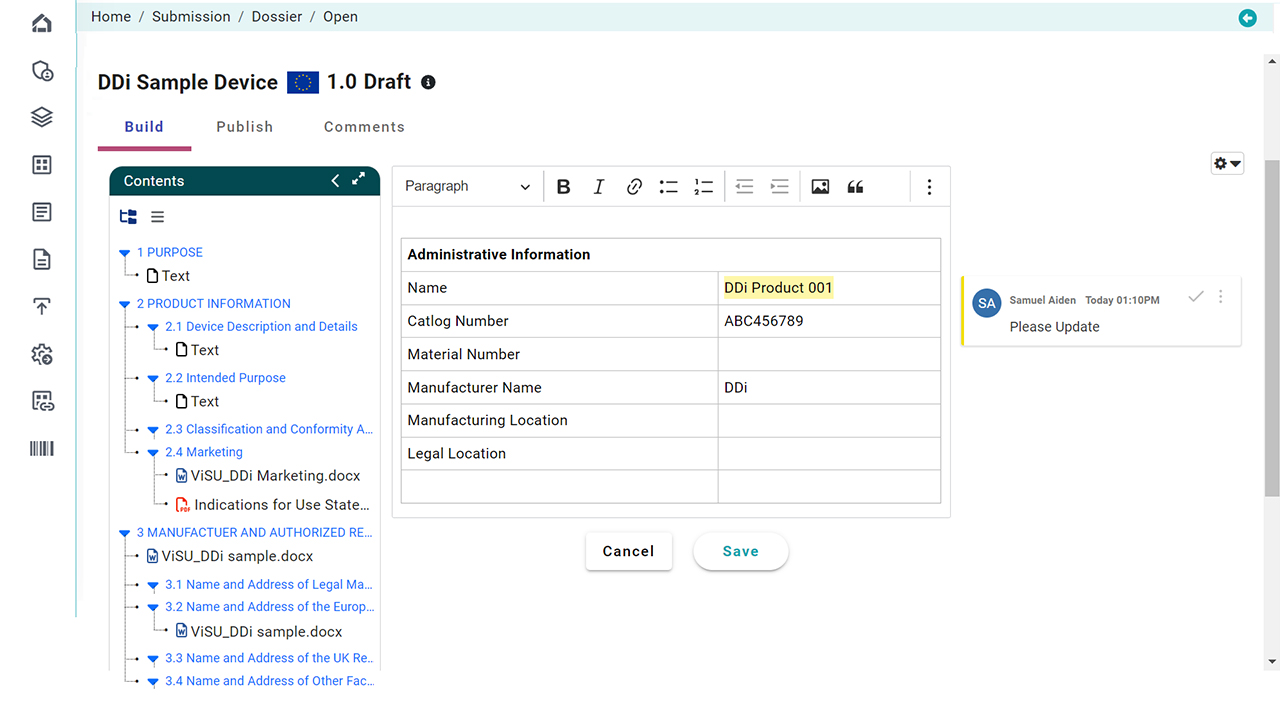
Document Collection: Regulatory operations publishing functionality manages collection of documents from various teams (or external systems we can connect to). These documents can be in various formats, such as Microsoft Word or Adobe PDF. Our built-in document format automation will QC and Auto-Fix documents as per country specific requirements
-
Document Preparation: The collected documents are then prepared according to the regulatory requirements and guidelines. This involves formatting, reviewing, and editing the documents to ensure consistency and accuracy. And our tool will handle all these steps as part of document automation process to save your time.
-
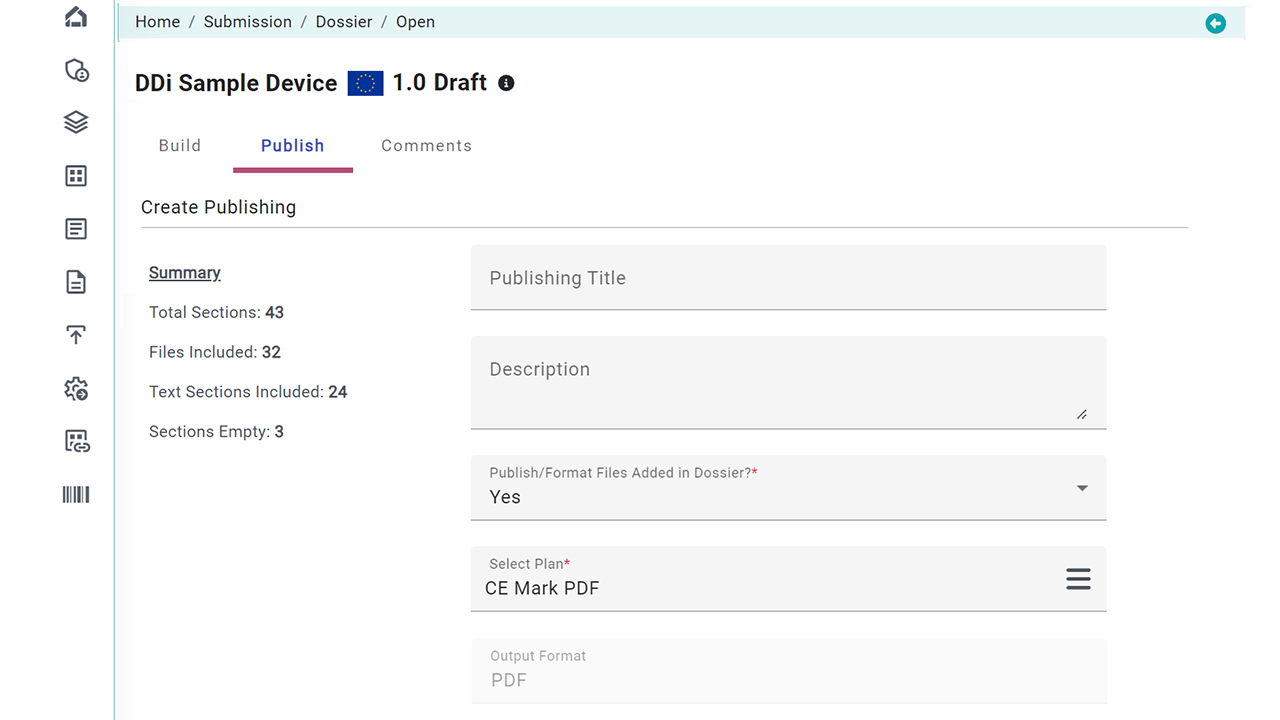
Document Publishing: The prepared documents are then converted into a submission-ready format (using our built-in “submission ready” functionality), and compiled into a submission package. This package is then published to the appropriate regulatory authority’s expectations
-
Submission Tracking: Regulatory operations publishing also involves tracking the status of the submission with the regulatory authorities. This involves monitoring for acknowledgments, requests for additional information, and other communication.
-
Life-Cycle Updates: Once the submission has been approved, regulatory operations publishing also includes updating the regulatory authorities with any changes to the product, such as label updates or manufacturing changes. Our detailed life-cycle management handles both document level operations and correspondence aspects
-
Compliance: Regulatory operations publishing must comply with regulatory requirements, such as the International Council for Harmonization (ICH) guidelines, regional regulations such as FDA regulations, EU MDR and your internal rule books as well as standard operating procedures (SOPs).
-
Collaboration: Regulatory operations publishing requires collaboration with internal and external stakeholders, including regulatory affairs, quality assurance, medical affairs, and clinical development teams. Our built-in submission project management and collaboration ensures that the regulatory submission is accurate, complete, and compliant.
Overall, regulatory operations publishing is critical and our solutions help ensure save time/costs, cut cycle time and maintain compliance.
Document Level Publishing
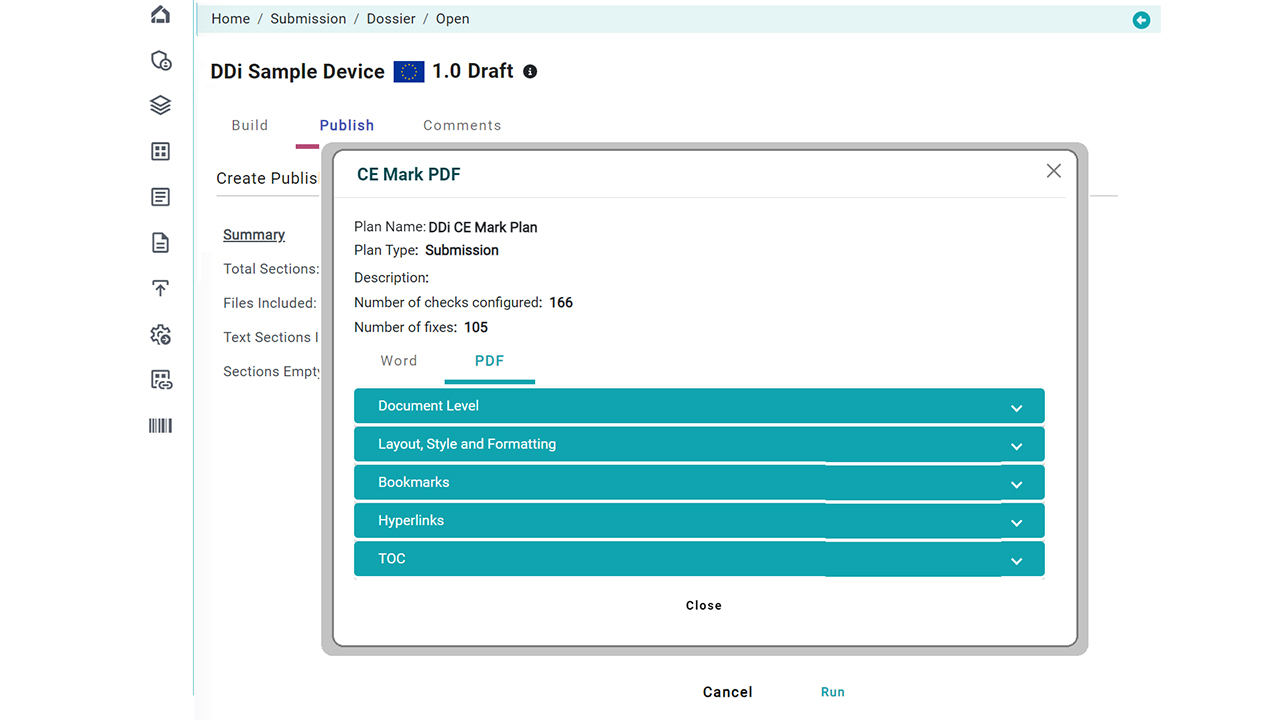
Publishing is Pain. Doesn’t matter what tools you use. Our solution automates document publishing. Whether your source files are in WORD DOC or PDF, get submission ready files in SECONDS. With 300+ rules library, you pick and create your custom plans with flexibility of applying what/when to your documents on the fly. You are in control as all this is done using a simpler user interface that’s cloud-based (or can be run as batch connecting to your EDMS or PLM/ERP systems).
ROI / Savings: 70% and above compared to your current costs. Guaranteed!
Functionality
- Publishing Rules: Standard Out-of-box rules for FDA, EU, and other regions +you can use any custom publishing rules from our vast library on DOC or PDF (Tables, Font management, TOC, Bookmarks, Headers/Footers, Hyperlinks, File-level general options, and more)
- File Sources: Upload from your Desktop or use our pre-built connectors to SharePoint, Documentum, BOX, and other 3rd party Sources.
- Download: Upload from your Desktop or use our pre-built connectors to SharePoint, Documentum, PLM/ERP, and several 3rd party Sources. The use of regulatory analytics can help regulatory teams to make more informed and data-driven decisions, leading to more effective and efficient regulatory policies and compliance.
- Access: Cloud (hosted on Private Cloud in USA/Germany)
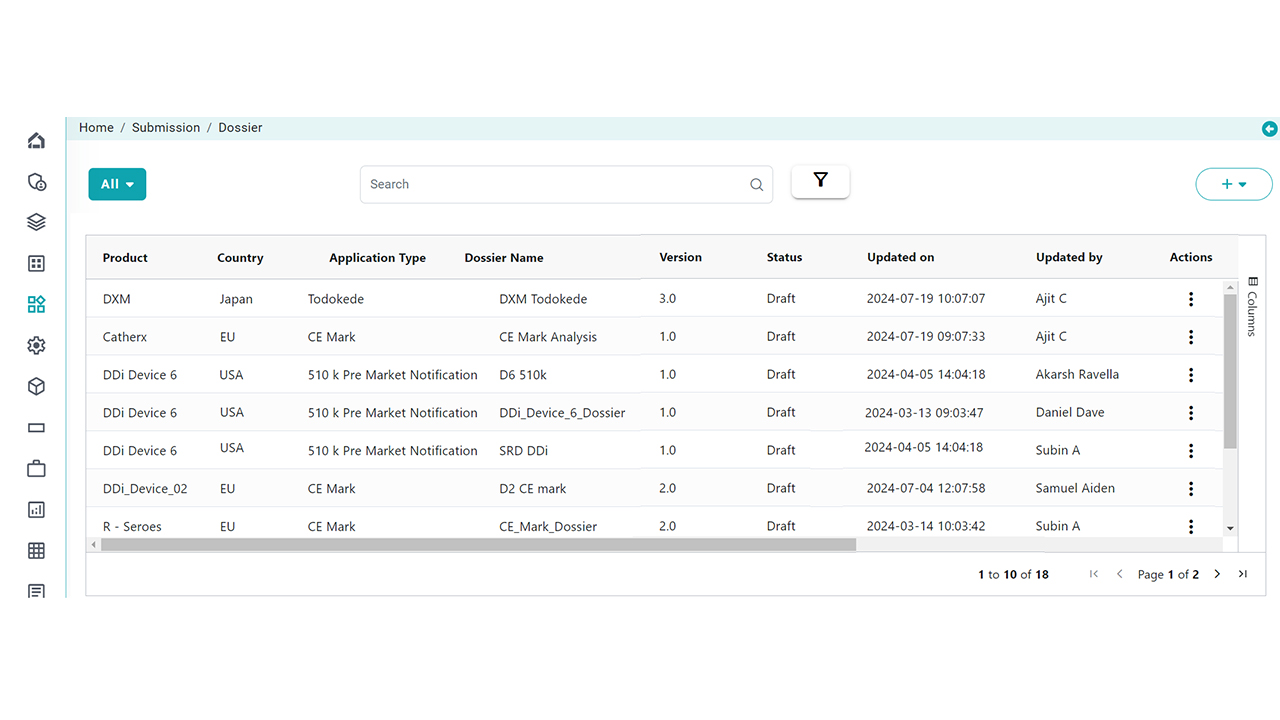
Dossier Build & Publish
Our solution enables you to readily build, view, validate and publish compliant submissions based on Standard country formats. Countries with custom submission requirements and all life cycle submissions of different applications for various countries. Some Highlights include:
-
Pre-built country publishing Plans
-
Custom Submission plans using our Template Management functionality
-
Centralize all publishing activities
-
Cross-country dossier linkages
-
Different outputs (XML, Zip, PDF)
-
Manage Life cycle easily
-
Integrated with your current tools/systems
We’re Here To Help
Get in touch with us
Let's talk about how DDi can help you


