
Why sponsors should opt Configurable IRT system, before opting any custom version?
Clinical trials are more complex than ever and a robust, reliable & cost efficient clinical supply strategy is vital more than ever before. The number and complexity of issues affecting supply chains have also grown to include costly comparative drugs, intricate protocols, delicate investigative compounds, adaptive clinical trials, variable dosing schemes increased regulation and country-specific approvals surrounding temperature-controlled drugs, packaging, shipping, and labeling and other factors.
As such 60-70% of the overall studies are simple, opting Configurable IRT version is crucial both in terms of timelines & pricing aspects. Usually, the normal practice would go this way, i.e., any given IRT vendor usually takes the specifications from a sponsor and tries to build customized IRT system which involves programming and this is the area where extra pricing would be added; which is unnecessary.
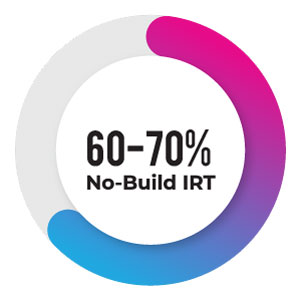
mIRT – Xpress version, DDi’s proprietary IRT offering ensures all setup is in front-end UI, which drastically reduces testing, setup time and UAT areas, also ensuring 70% faster setup than other products from other vendors.
With this configurable version, sponsors get the option of using the system in the conduct phase as well. And any changes in the system can be done in the front-end UI itself, unlike most of the IRT systems which doesn’t allow this feasibility option of sponsor team taking charge of IRT system.
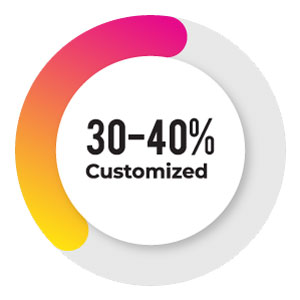
The remaining 30-40% of the studies which are usually complex might need customization, but even in this case any IRT vendor should first check whether this complex study can fit-in to the configurable version (which doesn’t happen in the real case scenario) and then plan accordingly as to what are the functionalities that need to be built.
DDi’s mIRT Xpress version is constantly in improving phase by getting added with various modules or functionalities into it, during every set interval of time; these modules or functionalities are available during the process of IRT customization.
For Small-Mid Pharma/Biotech companies, anyhow configurable version is best suitable both in terms of Cost & timeline aspects; even Large Pharma/ Biotech companies can get benefited immensely.
For companies looking for sophisticated randomization and clinical supply chain management solutions, clinical supply management tool, mIRT (Xpress & Custom) brings in greater value through its interactive response technology offering voice and web-based system in an integrated platform.
Get the latest updates from DDi
Explore Topics
- Automation & AI (2)
- Clinical Automation (8)
- Consumer Health (1)
- IRT & Clinical Supplies (19)
- Labeling (16)
- Regulations (21)
- Regulatory Automation (14)
- Regulatory Biopharma (2)
- Regulatory Content Management (5)
- Regulatory Information Management (13)
- UDI (11)
- Writing (11)
Recent Blogs
 Pharma and Biotech RIM Strateg…Regulatory Information Management
Pharma and Biotech RIM Strateg…Regulatory Information Management Non-Clinical Writing: Automati…Writing
Non-Clinical Writing: Automati…Writing Automation in Medical Writing:…Writing
Automation in Medical Writing:…Writing
Previous Post
Next Post
Related Posts
CONNECT WITH US

Let's talk about how DDi can help you




