Technical Documents Writing tools
While some documents like a Risk Management, Biocompatibility, R&D and few others are truly technical in nature, other documents like DOC, GSPR, CER, PMSR, PSUR and their life cycle changes involve re-usable content and data (like product information) and authoring tools help managing this re-usability can improve efficiency & save time of technical content authors in med device companies
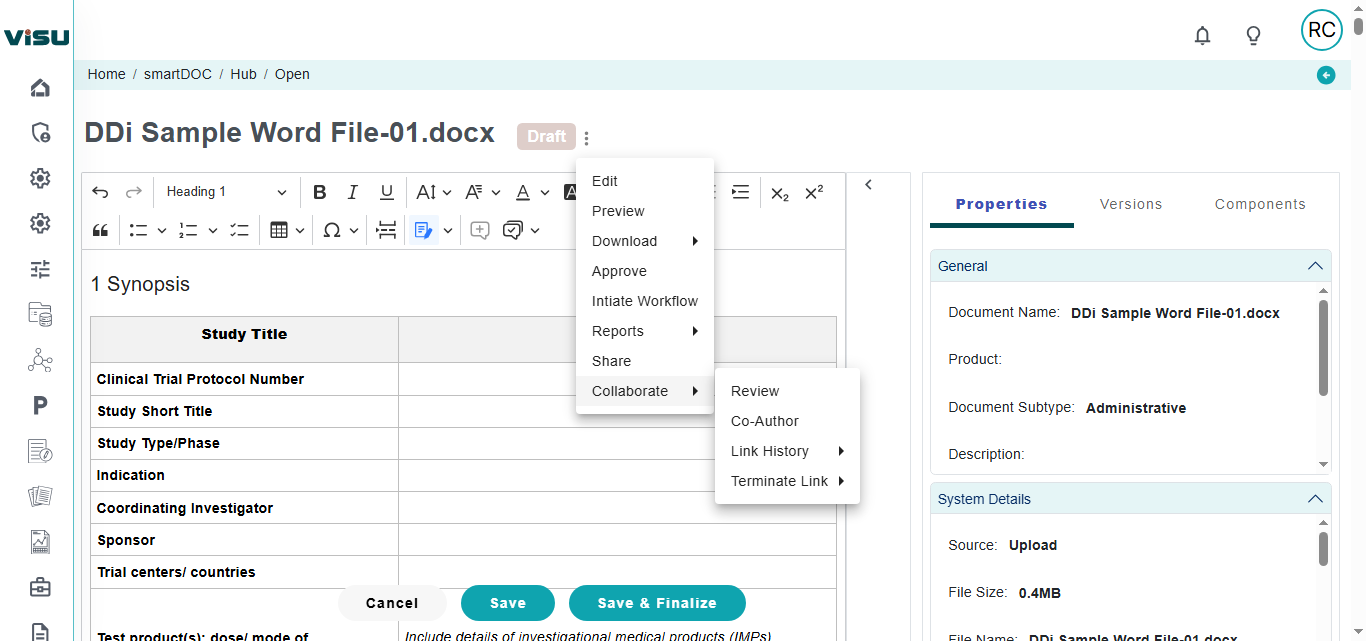
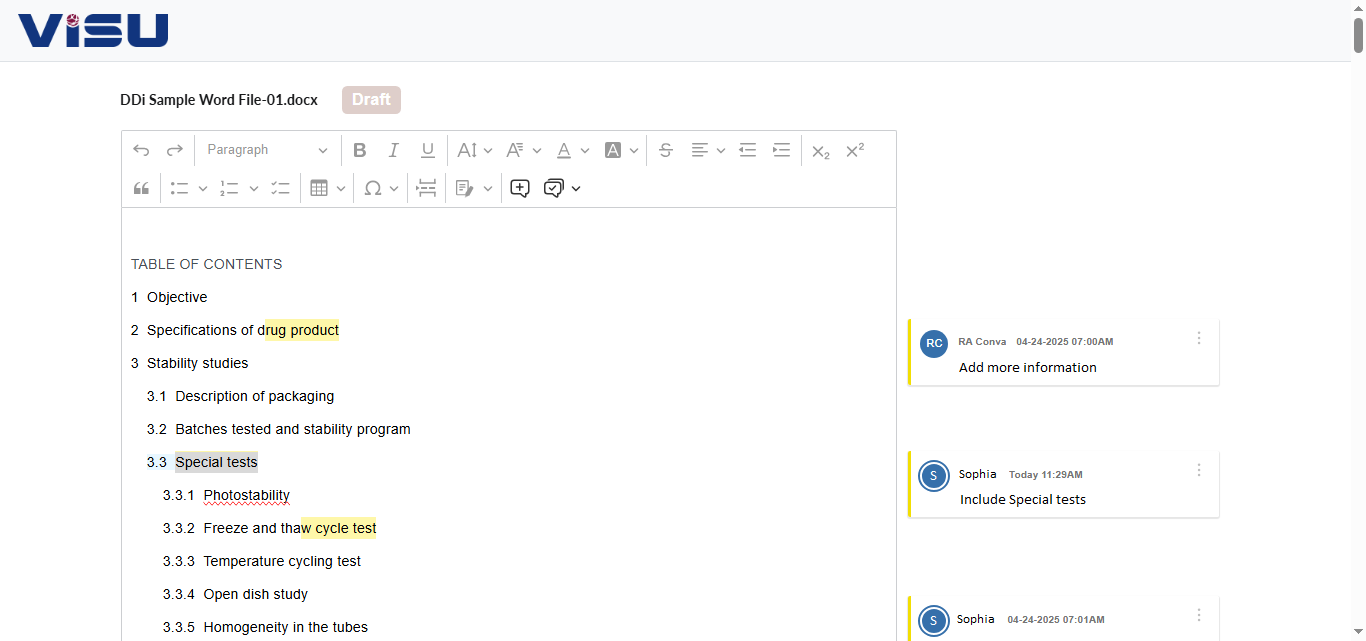
Writing UI: Use MS Word or our web based UI for authoring. As some of them need deep collaboration, you will have options to add workflows, QC, alerts and trackers. Central re-usable content repository can be leveraged both form MS Word and our Web based Writing interface
Data: Data (from change control, or product databases or master data or Excels) needed for some of your documents, pull and add directly into Documents using variables or data grids functionality in the tool. When source data changes, update the documents automatically without again having to redo most of the content
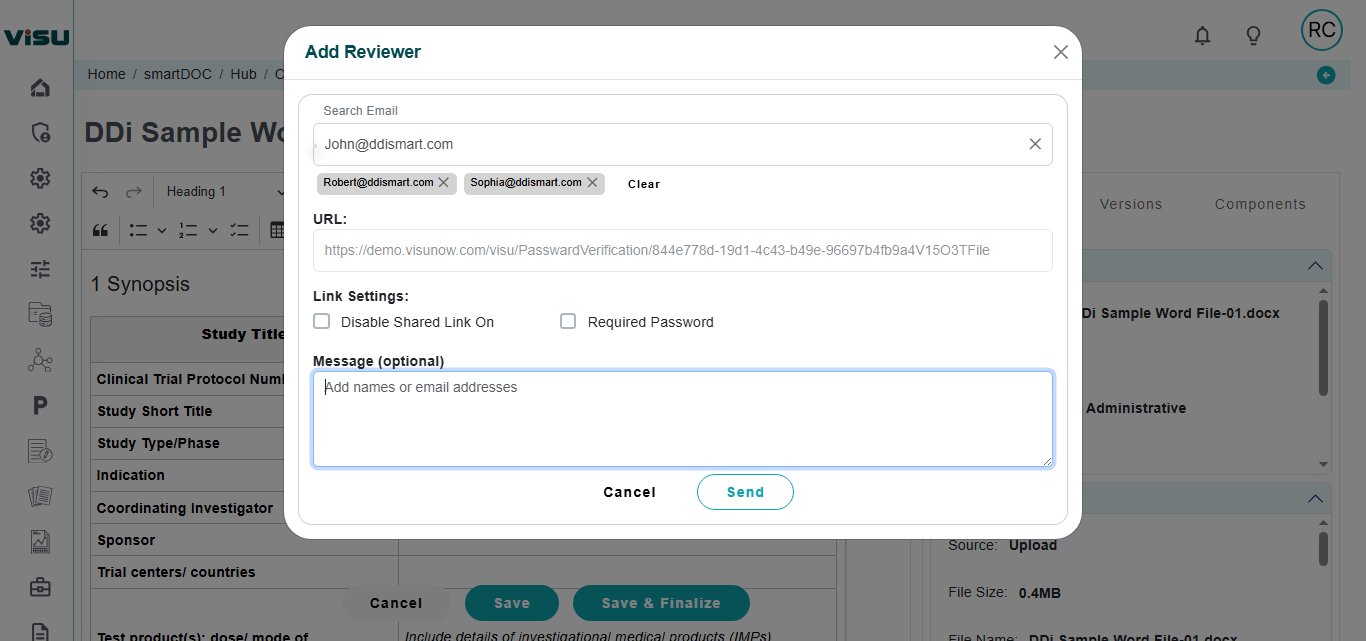
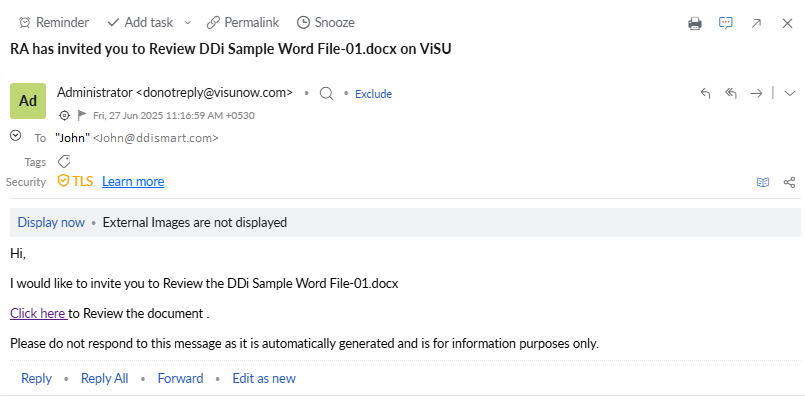
Collaboration: Full-fledged online collaboration included for internal/external stakeholders to co-author or review. Collaborate with users who do not have access to system as well. Consolidate comments
Formatting/Publishing: Reduce your formatting pain (and save your time) by leveraging auto-formatting functionality (450+ checks Word/PDF library) to get your submission ready output files in minutes which will take your rule books and style guides into consideration while delivering Word or PDF outputs.
Templates: Robust template management incorporating several Libraries to standardize structure. Import word templates or create your templates with drag/drop features.
We’re Here To Help
Get in touch with us
Let's talk about how DDi can help you

